JAFRAL offers production of GMP and non-GMP phages.
JAFRAL can develop a complete production process or optimize an existing one, for both lytic and filamentous bacteriophages.
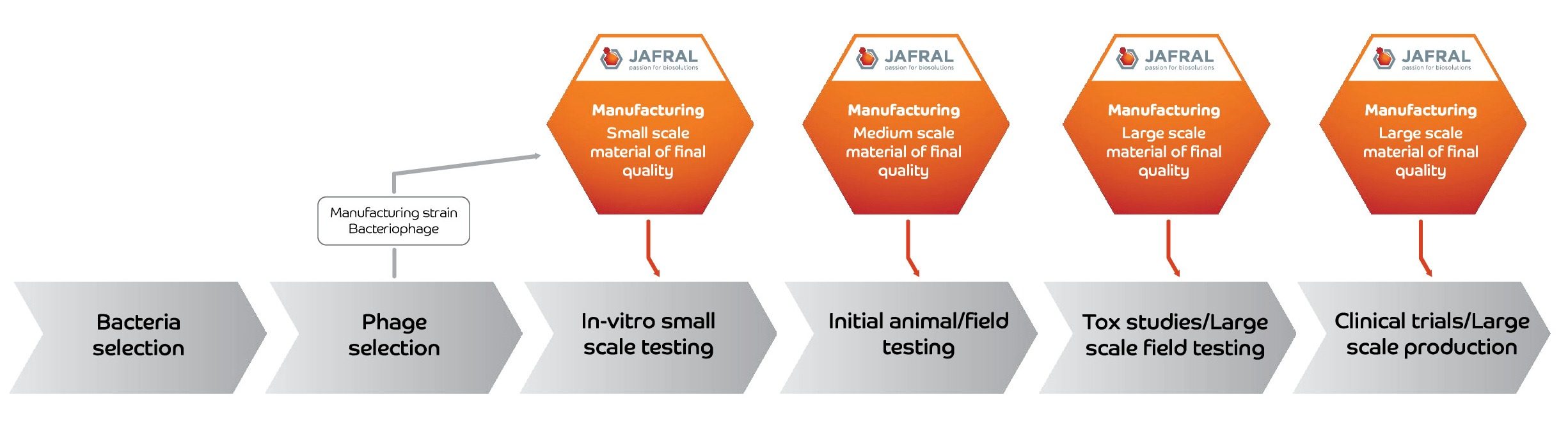
Our experience with different phages enables us to develop and optimize processes from petri dish to bioreactor and from lab scale purification to pilot scale production.
Optimized manufacturing processes result in high phage titers, high yields, and high purity delivered in a cost-effective manner using state of the art technology with optimized upstream and downstream processes.
JAFRAL’s track record:
- Diagnostics:
- Listeria phages
- Food/Agriculture applications:
- Listeria phages
- Salmonella phages
- Campylobacter phages
- Cosmetics:
- Propionibacterium phages
- Probiotics:
- Escherichia phages
- Veterinary:
- Listeria phages
- Salmonella phages
- Pseudomonas phages
- Human therapeutics:
- Escherichia phages
- Acinetobacter phages
- Pseudomonas phages
- Staphylococcus phages
- Clostridium phages
…and more!

Process activities
- Possible deliverables: crude phage lysate, concentrated crude phage lysate, purified concentrated phage material, various intermediates
- Upstream scale: erlenmeyer flasks, 1L, 15L, 50L, continuous
- Downstream scale: downstream capabilities match the scale of fermentation. This means that one fermentation batch can be purified in a single run without the need for sub-lotting
- Downstream options: IEX (Q, S), gel filtration, diafiltration, affinity and others