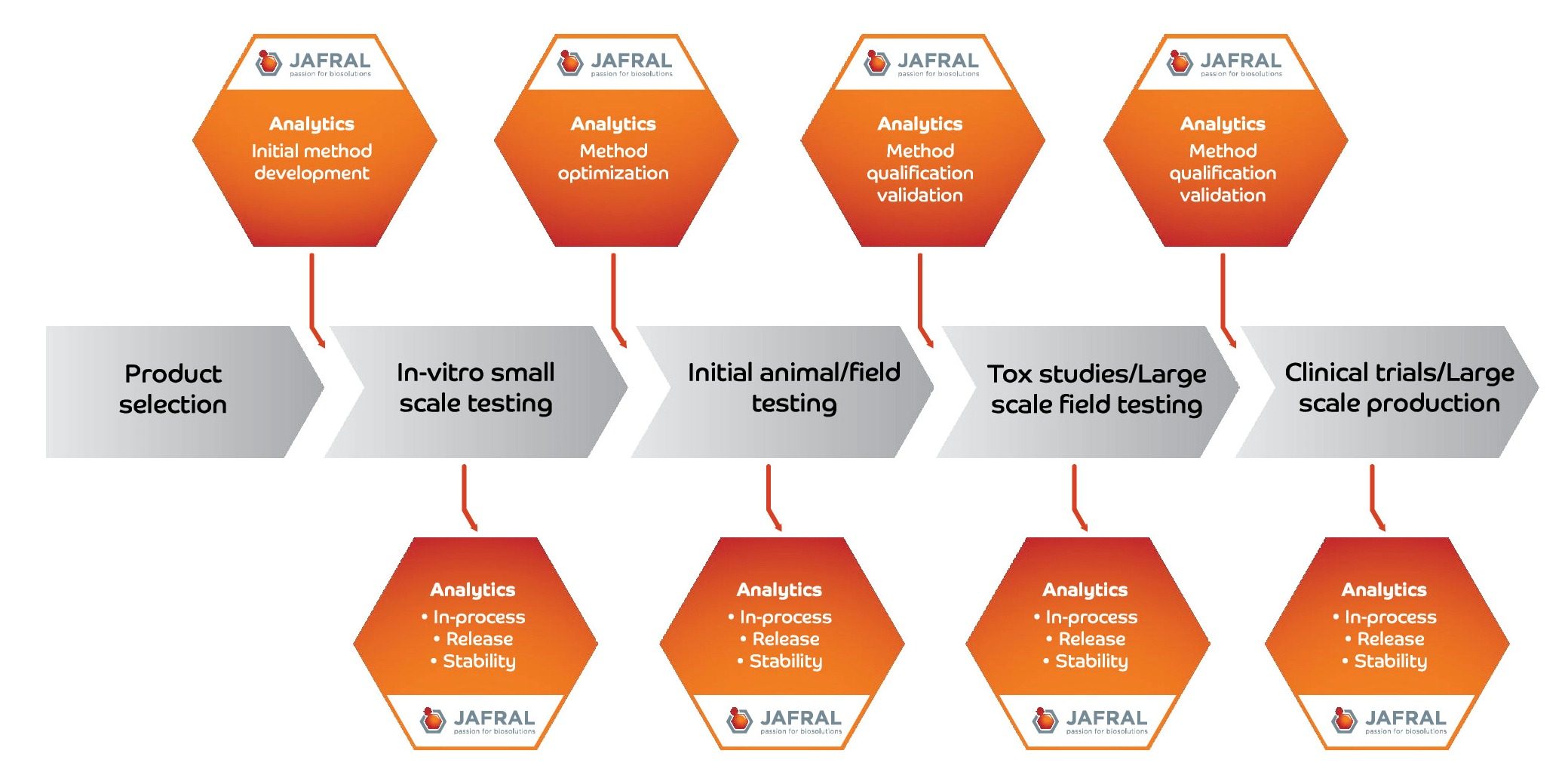
Development of Analytical Methods
JAFRAL’s strong believe is that good analytics is pre-requisite for fast and prompt process development as well as successful routine production of biopharmaceutical product.
Biotech companies face many hurdles along the way, including the development and optimization of a scalable, commercially viable formulation while ensuring safety and efficacy of the final drug product. In order to receive the approval of regulatory bodies, the manufacturing process and validation methods used to evaluate the drug product performance must be accurate and precise.
The analytical methods created during preclinical development play a major role of any regulatory filing. Robust and reproducible analytical methods can accelerate product and process development, which could lead to an early submission for regulatory approval and eventual product launch.

Analytical methods can be developed for various purposes:
- Incoming raw material testing
- In-process testing
- Release testing
- Stability testing
- Field trial, Animal trial samples
Example methods:
- Plaque assay (PFU/mL)
- Transducing forming units (TFU/mL)
- Bacterial counts,
- Endotoxin measurements,
- Identity,
- Measurements of A260/A280,
- Protein measurements and SDS-PAGE,
- DNA measurements
- HPLC/UPLC assays (SEC/RP/IEX)
- Immunoassays (Western Blot, ELISA)
- RT-PCR
- Etc.
Learn more about development of manufacturing process.